Autolus (NASDAQ:AUTL) is a CAR-T therapy developer with in-house manufacturing, multiple collaborations and a lead program that just met the endpoint in a pivotal trial in ALL. The company is UK-based. Its programs are described thus on Seeking Alpha:
The company’s clinical-stage programs include obecabtagene autoleucel (AUTO1), a CD19-targeting programmed T cell investigational therapy that is in Phase 1b/2 clinical trial for the treatment of adult ALL; AUTO1/22, which is in a Phase 1 clinical trial in pediatric patients with relapsed or refractory ALL; AUTO4, a programmed T cell investigational therapy for the treatment of peripheral T-cell lymphoma targeting TRBC1 and TRBC2; AUTO6NG, a programmed T cell investigational therapy, which is in preclinical trial targeting GD2 in development for the treatment of neuroblastoma; and AUTO8, a product candidate that is in a Phase I clinical trial for multiple myeloma. It also focuses on developing AUTO5, a preclinical TRBC2 programmed T cell product candidate for the treatment of peripheral T-cell lymphoma.
Lead program is obecabtagene autoleucel, a CD19 directed autologous CAR-T. It has been tested in R/R pediatric and adult B-ALL3 , as well as in other B-cell malignancies. The asset has met the primary endpoint in a pivotal study called FELIX in relapsed/ refractory adult acute lymphoblastic leukemia (ALL).
Obe-cel, the company says, is a CD19 binder that also has a fast off-rate. That means, while it binds to CD19, it also dissociates from the linkage quickly and has a shorter half life of interaction. To be specific, while Obe-cel takes 9.8 seconds for the binding and unbinding, Kymriah takes 21 minutes. Potentially, this has a positive impact on both efficacy and safety. Since these bindings are lossless, Obe-cel can quickly move on from one antibody to another, increasing proliferation, immune response and thus, effectiveness of a relatively lower dose of therapy. That, and the reduced persistence of linkage, also produces a better safety profile by avoiding over-activation of CAR T cells and reducing toxicities.
At ASCO and EHA 2023, Autolus presented data from FELIX. Here’s the link to the ASCO data. Here’s most of the presentation:
Methods: FELIX is an open-label, multi-centre, global, single-arm Phase Ib/II study enrolling r/r B-ALL patients with morphological disease (≥5% BM blasts), measurable residual disease (MRD) (≥0.1% to < 5% BM blasts), or isolated extramedullary disease (Cohorts A, B and C). CAR-T products were generated using an automated closed process from fresh leukapheresate. Patients underwent bridging therapy as necessary and lymphodepletion with fludarabine (4x30mg/m2) and cyclophosphamide (2x500mg/m2). Patients received a target dose of 410E+6 CAR T cells as a split dose on Day 1 and Day 10. The dosing schedule is based on the % BM blasts performed locally prior to the pre-conditioning. Primary endpoint was overall remission rate (ORR) defined as proportion of patients achieving CR/CRi by central assessment. Results: As of 9th September 2022, a pre-specified interim analysis was conducted based on the first 50 patients infused in Cohort A who have been followed for 3 months or discontinued before month 3. The median age was 50 years (range 20-81), 22% had Ph+ B-ALL. The median number of prior lines of treatment was 2 (range 1-5), 42% underwent prior transplant. At screening, patients had a median of 55% BM blasts (range 6-96%) and 26% had EMD. The geometric mean of peak CAR expansion was 126147.6 copies/ug genomic DNA. Persistence was ongoing in majority of responders at last follow-up. Based on central assessment, the CR/CRi was 70% [95% CI: 55%, 82%] (p-value < 0.0001). As of 9th September 2022, a total of 92 patients received obe-cel and were evaluable for safety. 63% developed any grade CRS (3% Grade ≥3) at a median of 9 days post-infusion and a median duration of 5 days. Any grade ICANS was observed in 23% (8% Grade ≥3) at a median of 15 days post-infusion and of median duration 8 days. Other common Grade≥3 adverse events regardless of causality were febrile neutropenia (25%) and anaemia (20%). Conclusions: The pre-specified interim analysis of the FELIX study demonstrated that obe-cel for adult r/r B-ALL is safe with low rates of Grade >3 CRS and/or ICANS, even in patients with high burden disease. Obe-cel is effective with high CR/CRi rates and ongoing CAR T persistence in the majority of responders. The trial has completed dosing of all patients in Cohort A. Additional data and longer follow up will be reported at the conference.
Thus, there was a 70% CR rate and a manageable safety profile in treatment-experienced patients who had undergone multiple lines of therapy. Duration of response, a key positive criteria for an expensive, late line therapy, was also good, with 61% of responders under remission at 9.5 months of follow up, needing no other cancer therapy.
In its early years, AUTL went through a few issues that delayed the stock’s progress. In 2019, for example, it let go of a lead program because the competitive space became crowded, and that particular program just couldn’t compete. In the same year, it faced manufacturing delays because a UK-based plant where it had leased space (or capabilities) could not deliver. It faced funding issues because some of its funding was tied to Neil Woodward’s ventures, which were facing existential threats at that time.
The present obe-cel data shows that the company has put these things back, and has arrived. While Gilead’s CD19 directed autologous CAR-T therapy Tecartus is already approved for ALL, AUTL’s claimed differentiation is a better safety profile and reduced T-cell exhaustion. As the company points out:
Current T cell therapies for adult patients are Blincyto® and TecartusTM – Both therapies are highly active, but frequently followed by subsequent treatments (e.g. alloSCT)
– Blincyto®: favourable safety profile, few patients experiencing severe CRS and ICANS, but limitations on convenience – continuous i.v. infusion during 4-week treatment cycles
– Tecartus™: more challenging to manage – induces elevated levels of severe CRS, a high levels of severe ICANS, and requires vasopressors for many patients
Thus, each of these two products has limitations which obe-cel addresses. Here’s another safety comparison chart versus SoC:
obe-cel vs SoC (autolus website)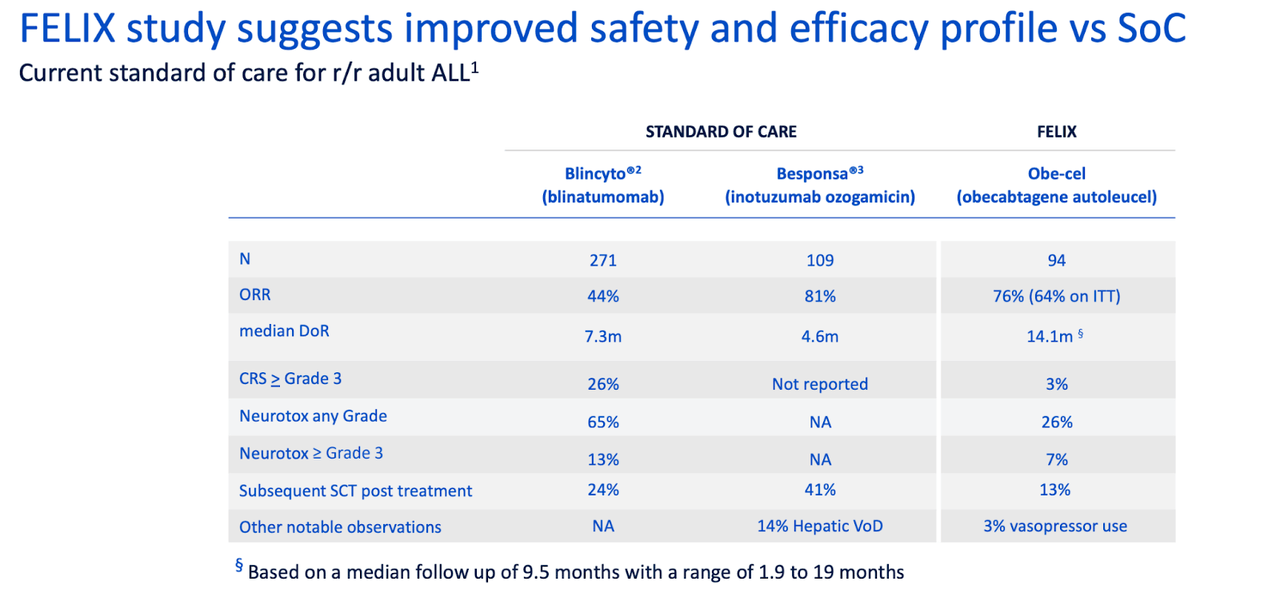
Obe-cel’s only problem is that it is open label and single arm, but autologous CAR-T therapies have that problem because of the difficulty of manufacturing, which precludes a comparator arm. The company is now planning a BLA by the end of the year.
Financials
AUTL has a market cap of $531mn and a cash balance of $308mn. Research and development expenses decreased by $1.5 million to $36.7 million for the three months ended June 30, 2023, while general and administrative expenses increased by $2.8 million to $11.1 million. At that rate, the company has a cash runway of some 6-7 quarters.
PE/VC firms and institutions hold most of AUTL. Key holders are Syncona, Blackstone, and Qatar Investments.
Bottomline
AUTL seems attractive right now, with a long cash runway and a superior product candidate with near term catalysts. I would go for a pilot position.
Read the full article here




